Stanford Researchers Reverse Autism-Like Behaviors in Mice Through Targeted Brain Modulation
Stanford Scientists Reverse Autism-Like Behaviors in Mice Through Targeted Brain Modulation
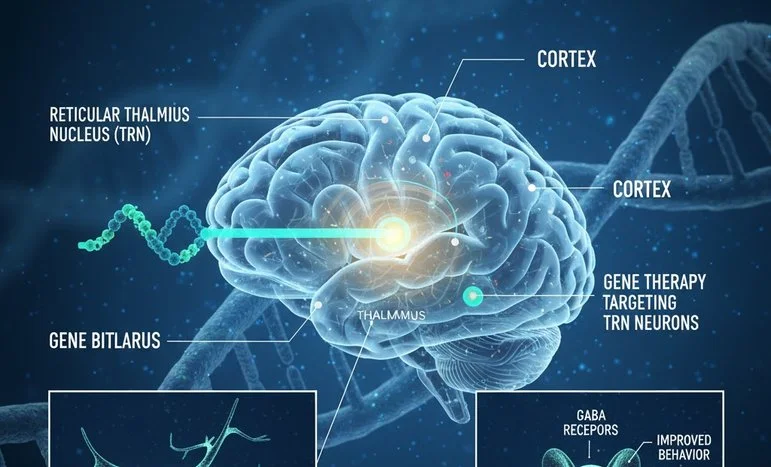
Researchers at Stanford University have made a groundbreaking discovery in neuroscience by successfully reversing autism-like behaviors in mice. The team achieved this by suppressing hyperactivity in the reticular thalamic nucleus (RTN), a crucial area in the brain that regulates sensory and cognitive processes. This study not only advances our understanding of the neural circuits underlying autism spectrum disorders (ASD) but also points toward potential therapeutic strategies for humans in the future.
The Role of the Reticular Thalamic Nucleus
The RTN is a thin shell of inhibitory neurons surrounding the thalamus. It acts as a control hub for sensory input and cognitive signaling, filtering information before it reaches the cortex. Dysregulation in this area has been linked to repetitive behaviors, social deficits, and hyperactivity, all core features of ASD. Moreover, the RTN is involved in circuits implicated in epilepsy, which provides a valuable link for therapeutic strategies that could target multiple neurological conditions simultaneously.
By selectively reducing hyperactivity in the RTN, the researchers were able to normalize the flow of neural signals, leading to measurable improvements in behavior.
Experimental Methods
The Stanford team employed a combination of optogenetic and pharmacological techniques to modulate RTN activity in mice genetically predisposed to autism-like behaviors:
- Optogenetics: This technique allowed precise temporal control of neuron firing using light-sensitive ion channels.
- Pharmacological Modulation: Drugs were administered to dampen overactive RTN neurons without affecting surrounding regions.
Behavioral assessments revealed significant improvements across multiple domains:
- Social Interaction: Mice engaged more frequently in social exploration and grooming behaviors.
- Repetitive Behaviors: Compulsive or stereotyped movements decreased.
- Cognitive Flexibility: Performance improved in maze-based problem-solving tasks.
- Exploratory Activity: Overall engagement with new environments increased.
These results confirm that hyperactivity in the RTN contributes causally to autism-like behaviors in mice.
Intersection with Epilepsy Research
Interestingly, the techniques used in this study overlap with therapeutic approaches for epilepsy, which also target thalamocortical circuits to reduce abnormal neural excitability. This connection suggests that some shared neural pathways may underlie both ASD-like behaviors and seizure disorders. Targeting these circuits may provide dual benefits, opening possibilities for multi-faceted treatment approaches.
Translational Implications for Human Therapy
Although these findings are based on mouse models, they offer valuable insights for human applications:
- Non-Invasive Neuromodulation: Techniques like transcranial magnetic stimulation (TMS) or focused ultrasound could potentially modulate RTN activity in humans.
- Targeted Drug Development: Pharmacological agents aimed at RTN signaling pathways may offer novel treatment strategies for ASD.
- Early Intervention: Identifying and modulating hyperactive neural circuits during critical developmental windows could prevent the solidification of maladaptive behaviors.
The study emphasizes circuit-level interventions rather than focusing solely on neurotransmitter imbalances, marking a paradigm shift in neurodevelopmental therapy.
Ethical and Practical Considerations
Translating these findings to human treatments involves careful consideration of:
- Safety: RTN modulation must avoid disrupting essential cognitive or sensory functions.
- Precision: Therapies must selectively target hyperactive neurons without affecting overall brain network integrity.
- Heterogeneity: ASD manifests differently across individuals, requiring personalized approaches.
Despite these challenges, the research establishes a proof of concept for circuit-specific therapy, providing a roadmap for future human trials.
Future Directions
The Stanford team is planning additional research to:
- Examine long-term effects of RTN modulation on cognitive and social behaviors.
- Explore other brain circuits interacting with the RTN that contribute to ASD.
- Collaborate with clinical teams to develop translational approaches, such as non-invasive human trials targeting similar neural pathways.
- Integrate genetic, behavioral, and molecular data to better understand the interplay between neural circuits and observed phenotypes.
Key Takeaway
The Stanford study demonstrates that autism-like behaviors in mice can be reversed by targeting hyperactivity in the reticular thalamic nucleus. By leveraging insights from epilepsy research and focusing on neural circuits, this work lays the foundation for innovative, precision-based therapies for ASD and related neurological disorders in humans. The findings mark a significant step forward in neuroscience, offering hope for effective interventions that could improve the lives of millions affected by developmental brain disorders.
We appreciate that not everyone can afford to pay for Views right now. That’s why we choose to keep our journalism open for everyone. If this is you, please continue to read for free.
But if you can, can we count on your support at this perilous time? Here are three good reasons to make the choice to fund us today.
1. Our quality, investigative journalism is a scrutinising force.
2. We are independent and have no billionaire owner controlling what we do, so your money directly powers our reporting.
3. It doesn’t cost much, and takes less time than it took to read this message.
Choose to support open, independent journalism on a monthly basis. Thank you.